
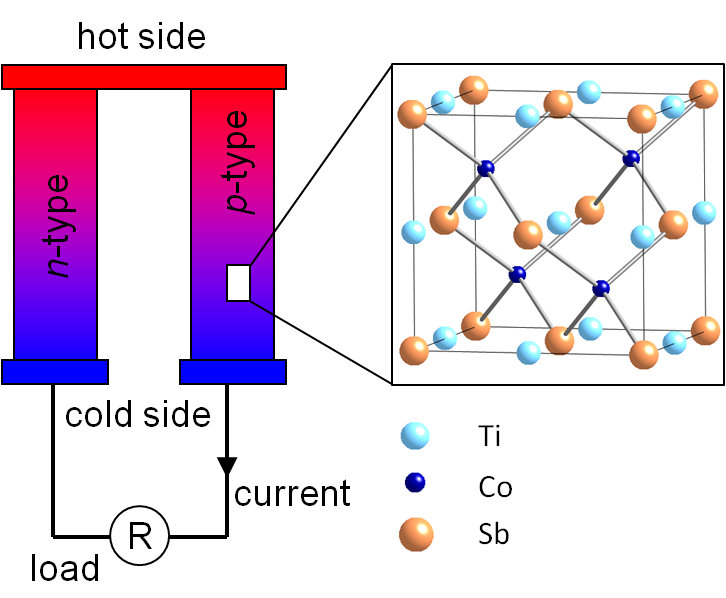
Schematic of a thermoelectric device consisting of a p- and n-type leg. The p-type leg is made up from the intermetallic material TiCoSb.
Thermoelectric waste heat recovery uses p and n-type semiconductors and a temperature gradient to generate electricity. A schematic module consisting of a single p- and n-type "leg" is shown in the figure above. The efficiency of this process depends on the temperature difference between the hot and cold side (the larger the better) and the energy conversion efficiency of the semiconducting materials. Our aim is to design, synthesise and characterise better performing thermoelectric materials. However, this is not a trivial endeavour as the efficiency depends on three materials parameters that are related through the electronic structure. This means that they cannot be optimised independently, and this limits the overall performance. These materials parameters are the Seebeck coefficient (the voltage response to a temperature gradient, the electrical conductivity and the thermal conductivity, which has an electronic and lattice part. In fact, only the lattice part can be manipulated independently from the the other variables, and phonon engineering is therefore a primary target in thermoelectric materials research. Typical current thermoelectric efficiencies are <10%. A good web resource is the Northwestern University Thermoelectrics website.
Most of our work has focussed on semiconducting Zintl-type intermetallic materials, including the half-Heusler structure indicated in the figure above. These materials are semiconducting despite only containing metallic elements and contain ionic and covalent regions, which offers good opportunities to reduce the lattice thermal conductivity without adversely modifying the conducting covalent regions. Materials containing transition metals often contain dispersive bands based on s-p hybridisation and localised states derived from the d-bands, which are the kind of features in the electronic band structure that are often linked to good thermoelectric performance.
We are also interested in transition metal oxides. These materials are quite different, whereas the intermetallic materials are good semiconductors with high mobilities (little charge carrier scattering), oxides are characterised by much lower mobilities but larger carrier concentrations. In general, transition metal oxides don't support the same kind of outright performance but they have advantages in terms of cost, stability at high temperatures, and there is experience with this kind of materials in the semiconductor industry.
Heriot-Watt University, Scottish registered charity number: SC000278